How New Drugs Are Developed: The Journey from Discovery to Approval
How new drugs are developed? The development of new drugs is a complex, multi-phase process that involves years of research, testing, and regulatory oversight. Each stage in the process is designed to ensure that new medications are safe, effective, and ready for widespread use. From initial discovery to regulatory approval, bringing a new drug to market is a monumental scientific and financial undertaking.
Step 1: Drug Discovery
The journey of a new drug begins with drug discovery, where researchers seek to identify potential candidates for treating specific diseases or medical conditions. This phase can span several years and typically involves the following steps:
-
Target Identification and Validation
Scientists start by identifying a biological target that plays a role in the disease they are trying to treat. This target could be a protein, enzyme, gene, or receptor involved in the disease’s progression. Furthermore, Validation of the target ensures that modifying its function can produce therapeutic effects.
-
Compound Screening
Once a target is identified, researchers screen thousands of compounds to see if any interact with the target in a way that could lead to a therapeutic effect. This process can involve high-throughput screening, which uses automation to rapidly test large numbers of compounds.
-
Lead Compound Selection
From the screened compounds, the ones that show promise in interacting with the target are selected as “lead compounds.” These compounds will undergo further refinement to improve their efficacy, selectivity, and safety.
-
Preclinical Research before drug discovery
Before clinical trials can begin, the lead compound must be studied in preclinical models, typically involving lab animals. These studies aim to assess the drug’s pharmacokinetics (how the body absorbs, distributes, metabolizes, and excretes the drug) and its potential toxicity. In addition, researchers also identify the best dosage and administration methods during this phase.
Step 2: Clinical Trials for drug discovery
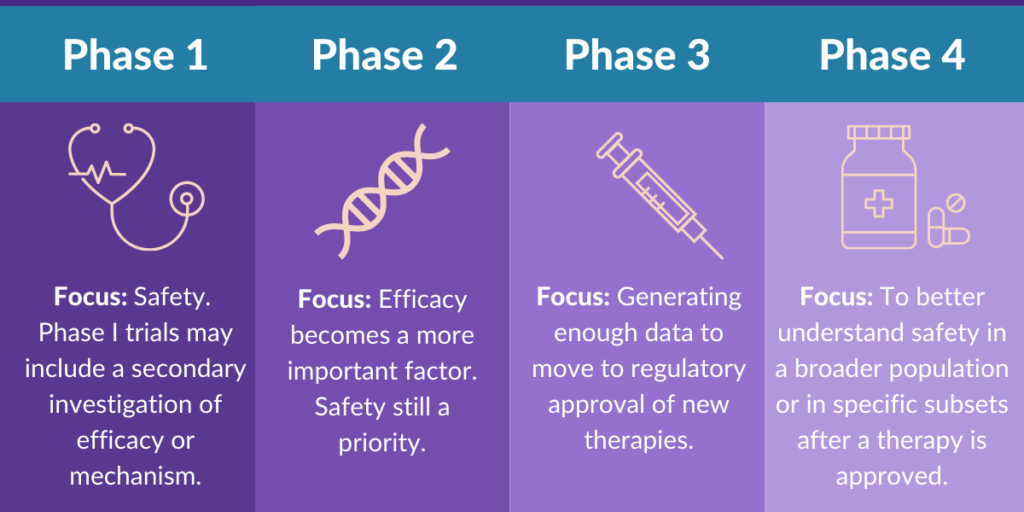
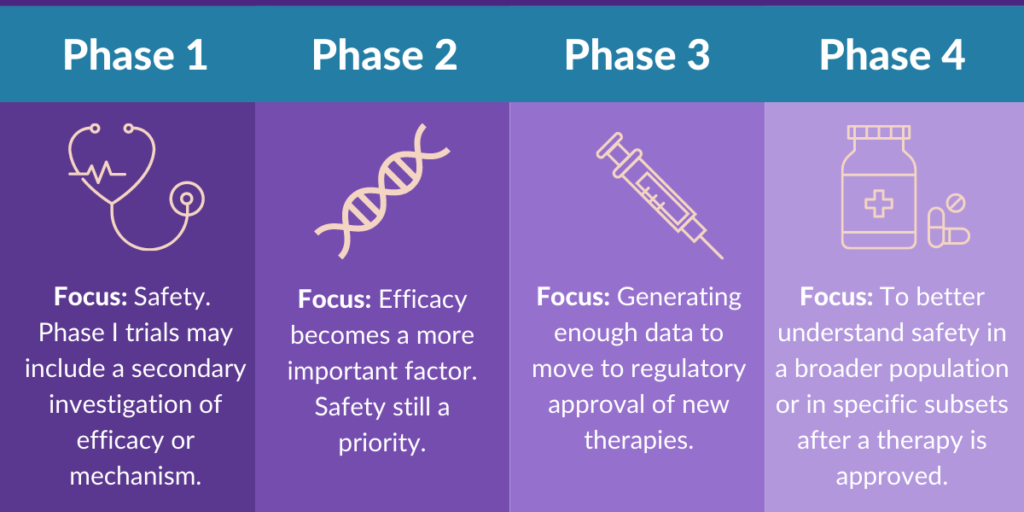
After preclinical testing, if the drug shows promise, it enters clinical trials, which are conducted in humans to assess the safety, dosage, effectiveness, and side effects of the drug. Clinical trials are generally divided into three phases: Phase 1, Phase 2, and Phase 3.
Phase 1: Safety and Dosage
The first phase of clinical testing involves a small group of healthy volunteers (usually between 20 to 100 people). As, the main goal is to determine the safety of the drug and establish the correct dosage range. As a result, researchers look for any adverse effects or side effects that might occur when the drug is administered.
Key objectives of Phase 1 trials include:
- Assessing how the drug is absorbed and metabolized in the body.
- Determining the safest dosage range.
- Identifying any immediate or long-term side effects.
Because Phase 1 trials typically involve healthy participants, they do not focus on whether the drug is effective in treating the disease. Instead, they provide foundational data about how the drug behaves in humans.
Phase 2: Efficacy and Side Effects
In Phase 2, the drug is tested on a larger group of patients (usually between 100 to 300) who have the condition the drug is intended to treat. The primary goal of this phase is to assess the drug’s effectiveness and further monitor its safety.
Key objectives of Phase 2 trials include:
- Testing the drug’s therapeutic effect on patients with the target disease.
- Monitoring adverse effects that may arise in a larger population.
- Fine-tuning the dosing schedule to optimize results.
This phase provides early evidence of the drug’s efficacy and helps determine the optimal dose and administration method.
Phase 3: Confirmatory Trials
Phase 3 is the largest and most comprehensive phase of clinical testing. It involves a much larger group of patients (typically several hundred to several thousand) and is designed to confirm the drug’s effectiveness, monitor its side effects, and compare it to existing treatments. Researchers conduct randomized controlled trials (RCTs) to ensure the results are as reliable as possible.
Key objectives of Phase 3 trials include:
- Confirming the drug’s clinical benefits.
- Assessing its long-term safety and effectiveness.
- Comparing it to current treatment options.
- Monitoring any rare side effects or long-term effects.
Phase 3 trials are critical in establishing the therapeutic benefit of a drug and determining its risk-benefit profile. If the drug performs well and meets safety standards, it can move on to the regulatory review process.
Step 3: FDA Approval Process
Once clinical trials are successfully completed, the drug’s developer submits an application to the U.S. Food and Drug Administration (FDA) for approval. Moreover, the FDA is responsible for evaluating the safety, efficacy, and quality of drugs before they can be marketed to the public. Hence the approval process is rigorous and can take several months to a few years, depending on the complexity of the drug and its clinical trial data.
New Drug Application (NDA)
To seek FDA approval for new drug discovery, a pharmaceutical company submits a New Drug Application (NDA), which contains detailed information about the drug’s:
- Chemical composition.
- Manufacturing process.
- Preclinical and clinical trial data.
- Proposed labeling and instructions for use.
- Risk-benefit analysis.
In addition, the NDA must demonstrate that the drug is safe and effective for its intended use, based on the results of clinical trials.
FDA Review Process to approve drug discovery
Once the NDA is submitted, the FDA’s team of experts, including physicians, chemists, and statisticians, thoroughly reviews the data. The review process typically includes:
- Clinical Review: The FDA evaluates the results of clinical trials, looking at both the drug’s benefits and risks.
- Safety Assessment: The FDA ensures that the drug is not likely to cause harmful side effects, considering both common and rare adverse events.
- Labeling Review: The FDA checks the proposed labeling to ensure that it provides clear instructions on how to use the drug safely and effectively.
The FDA’s review of the NDA usually takes around 6 to 10 months, though this timeline can be shorter for drugs that are designated as “priority review” or longer for more complex drugs.
Advisory Committees and Recommendations
In some cases, the FDA may convene an independent advisory committee to review the data and provide recommendations on whether to approve the drug or not. These committees consist of experts in the relevant therapeutic areas and may provide additional insights into the drug’s safety and effectiveness.
While it’s not compulsory to follow the committee’s recommendations, but during the decision-making process they take it as serious consideration.
Approval and Post-Marketing Surveillance
If the FDA determines that the drug is safe and effective for its intended use, it grants approval, allowing the drug to be marketed in the U.S. However, FDA approval does not mark the end of the drug’s journey. Even after approval, the drug enters the post-marketing phase, where it is monitored for any long-term or rare side effects. This phase is known as Phase 4 clinical trials.
Post-marketing surveillance includes:
- Adverse Event Reporting: Healthcare providers and patients are encouraged to report any side effects to the FDA or the drug manufacturer.
- Ongoing Studies: Some drugs may be subject to additional clinical trials to monitor long-term effects or explore new uses.
- Periodic Reviews: The FDA conducts periodic reviews of the drug’s safety profile and may require label updates or restrictions based on new findings.
Conclusion
The development of new drugs is a lengthy, highly regulated process that ensures patient safety while fostering innovation in medicine. From initial discovery through preclinical testing and clinical trials to FDA approval and post-marketing surveillance, each phase plays a critical role in bringing safe and effective treatments to the market.
Furthermore, the clinical trial process, in particular, is vital for establishing a drug’s therapeutic potential while safeguarding against unnecessary risks. With the rigorous evaluation by the FDA, patients can be confident that new medications meet the highest standards of safety and efficacy. While the journey from drug discovery to FDA approval is complex and costly, it is essential for advancing medical science and improving global public health.